Medical Device Design & Development
We design, build and iterate medical devices - mechanical, electronics and firmware - backed by measurements, user insight and documentation that stands up in review.
Where we help (at any stage)
New concepts that need realistic designs and fast iteration.
Prototypes that must become reliable, testable builds.
Existing designs that need performance, cost or manufacturability improvements.
Teams preparing for verification and design transfer.
Outcome: designs that work on the bench, make sense for users, and connect cleanly to verification and manufacturing.
Capabilities
Mechanical
Mechanisms, housings, drive trains, sealing, tolerance stacks, materials selection, DFM/DFA.
Electronics
MCU selection, sensor chains, power, drivers, quick PCBs; production handover with your EMS.
Firmware
Control loops, safety guards, logging, update strategy; 62304 alignment where required.
Human factors
Form studies, task analysis, formative sessions, usability risk linkage (IEC 62366).
How we run design
Architecture & risk
Define blocks, interfaces and constraints; keep ISO 14971 risk live and connected to decisions.
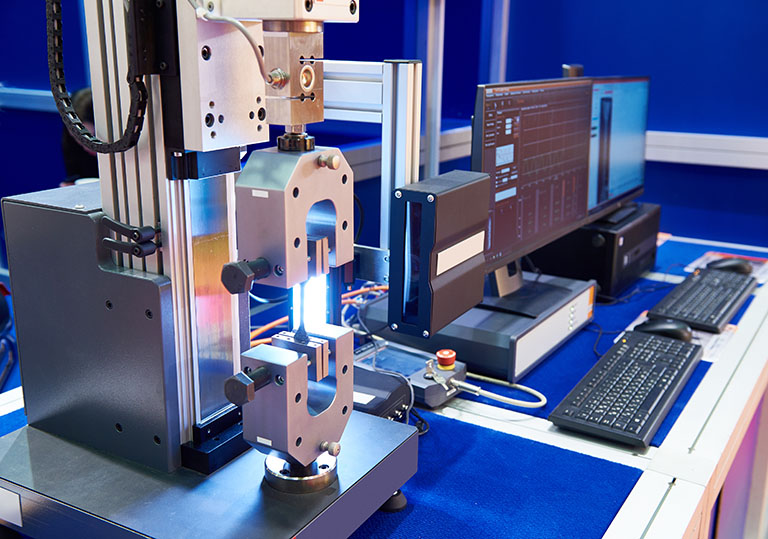
Build-measure loops
Design → fabricate → test → adjust; rigs and metrics that matter (force, flow, dose, repeatability).
User insight
Quick formative checks on handling, displays, controls, and maintenance tasks.
Evidence & documentation
Design inputs, decisions, traceability seed; what’s needed for verification later.
Design phases (pick where you need us)
Concept & feasibility – prove the mechanism and approach.
Prototype & iteration – reliable, testable builds with data.
Design for verification (DfV) – define characteristics and methods early.
Design transfer readiness – drawings, tolerances, materials, suppliers, QA hooks.
Deliverables
CAD and drawings (with versioning), simple BOM and spec sheets
PCB files/firmware where applicable; logging and test notes
Bench data and photos/video of runs
Design inputs and decision logs, traceability seed
A clear next-phase plan (verification or transfer)
FAQs
Either. We can own the design, embed with your engineers, or split responsibility by subsystem.
Yes for early to mid-stage development and production handover. We collaborate with your EMS and escalate specialists where needed.
Design inputs, risk and usability are kept live; we define verification characteristics early so evidence flows cleanly into the Technical File.
Yes – tolerances, materials, drawing packs, and supplier selection/briefing. We can run design transfer readiness as a separate phase.
As standard, all outputs are client-owned; we can work in your repositories if preferred.